Background information on Toxoplasma & other apicomplexan parasites
Toxoplasma & the Apicomplexa:
Toxoplasma gondii is a member of the Apicomplexa, a phylum of parasites that includes a number of medically and agriculturally significant pathogens. These parasites have a common ancestor and this is reflected in conserved morphological features (see Figure 1). Parasites of the Apicomplexa are named for their distinctly polarized cell apex that contains a number of unique organelles. These organelles (the rhoptries, micronemes, conoid and apical polar ring) are critically important for invasion and survival within host cells. Rhoptries and micronemes are unique secretory organelles that contain products required for motility, adhesion to host cells, invasion of host cells and establishment of the parasitophorous vacuole. The conoid is a small cone-shaped structure comprised of a spiral of novel filaments that is thought to play a mechanical role in invasion of host cells. The apical polar ring, a structure unique to apicomplexans, serves as one of the two microtubule-organizing centers (MTOCs) in these parasites. In addition to the apical complex, the Apicomplexa have other unique structural features, such as an essential chloroplast-like organelle called the apicoplast. Apicomplexan parasites are bounded by the pellicle, a composite structure consisting of the plasma membrane and the closely apposed inner membrane complex which is comprised of flattened vesicles.
 |
|||
| Figure 1: In addition to Toxoplasma, other apicomplexans illustrated here include: Lankesterella (parasites of birds and amphibians), Eimeria (parasites of cattle and chickens), Isospora (parasites of dogs and cats), Sarcocystis (parasites of hoofed animals), Babesia (parasites of cattle) and Plasmodium spp. (agents of malaria). Other apicomplexans include Cryptosporidium and Theileria (parasites of cattle) and Cyclospora (foodborn outbreaks in humans). Plasmodium spp. are established human pathogens, Toxoplasma and Cryptosporidium are opportunistic infections of immunocompromised individuals and other Apicomplexa such as Babesia and Isospora appear as zoonotic infections of humans. Electron micrographs used in this figure are from the elegant studies of Scholtyseck and Aikawa. For further information about parasitic diseases, see http://www.dpd.cdc.gov/dpdx/Default.htm. Apicomplexan parasites have a number of shared morphological features that are unique to the phylum. These traits include the apical complex organelles (the rhoptries, micronemes, conoid and apical polar ring). | |||
The apicomplexan life cycle:
Apicomplexans are haploid during most stages of their life cycle. The rapidly proliferating haploid parasite stages cause the acute and deleterious symptoms of infection. In addition to asexual mitotic proliferation, these parasites differentiate to gamete forms that fuse to make zygotes (Figure 2). Differentiation and fertilization occurs in the intestinal epithelia of appropriate host organisms. For example, Plasmodium gametogenesis and fertilization occur in the mosquito intestine whereas in Toxoplasma these events occur in the cat intestine. Fertilization of the immotile macrogamont by a flagellated microgamete creates a diploid zygote. The zygote undergoes meiosis to produce haploid sporozoites. In the case of Toxoplasma infection, sporozoites are shed in cat feces. In the case of Plasmodium infection, sporozoites migrate to the salivary glands of mosquitoes and are infectious to humans during the next blood meal feeding by the mosquito.
 |
|||
| Figure 2: For the faint of heart or those not interested in details, the center circle illustrates a generic apicomplexan life cycle. The rapidly proliferating haploid form has the capacity to differentiate into gametes that fuse to produce a diploid zygote. Meiosis re-establishes the haploid state and leads to sporozoites, the form associated with establishment of new infections. The outer two circles represent the specific life cycles for Toxoplasma gondii and Plasmodium falciparum. | |||
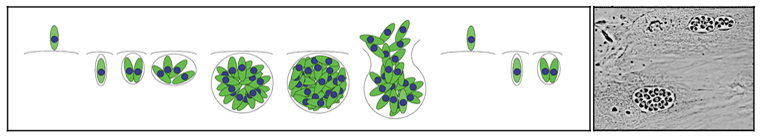
Apicomplexans are obligate intracellular parasites; these protozoa only grow and replicate within host cells. Extracellular parasites released by host cell lysis must rapidly invade new host cells in order to stay alive. Parasite replication occurs after invasion of a host cell, within a membrane-bound parasitophorous vacuole, and continues until the host cell is lysed by the replicating parasites. Extracellular parasites released by host cell lysis must find new host cells to invade to continue the cycle. This process is illustrated in Figure 3.
 |
|
| Figure 3: Parasite replication occurs after invasion of a host cell, within a membrane-bound parasitophorous vacuole. Eventually, the host cell is lysed by the replicating parasites. Extracellular parasites find new host cells to invade to continue to survive and replicate. | |
Parasite replication:
Apicomplexan parasites replicate by internal budding to create either two daughter cells or multiple progeny. Nuclear divisions occur without nuclear membrane breakdown and karyokinesis occurs without chromosomal condensation. Replication in Toxoplasma and Neospora occurs by endodyogeny, which creates two daughter parasites (Figures 4 & 5). Replication in Plasmodium, Theileria, Eimeria and Babesia occurs by schizogeny, which can create 64 daughter parasites (Figure 4). The processes of endodyogeny and schizogeny are quite similar, differing mainly in the preservation or loss of maternal cell specialization. In endodyogeny, two daughter parasites are formed within an intact, fully polarized mother parasite. This preserves the capacity of replicating parasites to invade throughout the cell cycle. The internal daughter cells are delimited by inner membrane complex and associated subpellicular microtubules and each contains (in addition to the nucleus, mitochondrion, Golgi and plastid) a complete set of apical organelles (conoid, rhoptries, micronemes) that are essential to invasion. When the daughter cells are fully mature, the maternal apical complex is disassembled and the daughters bud from the mother, adopting her plasma membrane. In schizogeny, after host cell invasion, the parasite subpellicular microtubules and apical complex are disassembled. After growth and multiple nuclear divisions, polarized parasites are regenerated when the nuclei move to the schizont periphery and associate with assembling inner membrane complex, subpellicular microtubules, and apical organelles. The newly re-polarized parasites then bud out of the maternal cell as merozoites. Due to their large round shape and lack of apical specialization, replicating parasites are non-invasive during schizogeny.
 |
|||||
| Figure 4: Endodyogeny proceeds without loss of maternal cell shape and apical polarity. Formation of a curved nucleus is coupled to construction of two buds composed of nascent daughter inner membrane complex and subpellicular microtubules. The daughter cells develop surrounded by their own subpellicular microtubules and inner membrane complex in the fully polarized mother cell. Once the daughter cells are mature, they bud out of the remnants of the mother cell. Schizogeny proceeds with loss of apical polarity. Invasion of a polarized elongated parasite is followed by disassembly of the subpellicular microtubules and inner membrane complex and with extensive cell growth and nuclear divisions. In order to reassemble polarized daughter cells, the multiple nuclei align with individual sets of apical organelles, subpellicular microtubules and inner membrane complex at the periphery of the replicating cell. | Figure 5: (Top) Immunofluorescence of Toxoplasma tubulin. Each crescent-shaped mother parasite (M) contains two daughter parasites (D) enclosed by individual sets of subpellicular microtubules. The maternal subpellicular microtubules and apical complex are retained so that parasites are competent for invasion throughout the cell cycle. (Bottom) Electron microscopy captures daughter parasites emerging from a maternal cell. In the outward-facing areas of the daughter cells, the dissociation of the maternal inner membrane complex from the plasma membrane is coordinated with association of the daughter inner membrane complex onto the plasma membrane (enlarged inset). Between the two daughter cells, scission involves membrane fusion events to create new plasma membrane (enlarged inset). | ||||
Apicomplexan parasites are bounded by the pellicle, a composite structure consisting of the plasma membrane and a closely apposed inner membrane complex which is comprised of a patchwork of flattened, Golgi-derived vesicles. The pellicle is intimately associated with a number of cytoskeletal elements, including actin, myosin, microtubules and a network of intermediate filament-like proteins. Apicomplexan protozoa share a number of cytoskeletal elements with other “typical” eukaryotic systems used to study the cytoskeleton (Figure 6). Nonetheless, studies of the cytoskeleton of apicomplexans have revealed startling differences from model organisms. For example, the singlet subpellicular microtubules of apicomplexans are unusually stable, and withstand the high pressure, cold and detergents used to isolate them, conditions incompatible with survival for most microtubules. In contrast, the microfilaments of apicomplexans are thought to be exceedingly transient. Microfilaments are only observed after treatment with jasplakinolide (a drug that drives actin polymerization) and the bulk of actin (>98%) is sequestered in globular form. Apicomplexan myosins are unconventional, constituting a new class of unusually small “neck-less” motors. Apicomplexan protozoa constitute an ancient and diverse phylum with peculiar cell biological traits that make these parasites an intriguing topic for study.
 |
||
|
Figure 6: (A) Subpellicular microtubules radiate out of the apical polar ring and run down the cytosolic face of the inner membrane complex while spindle microtubules originate in plaques embedded within the nuclear membrane. Atypical centrioles (consisting of nine singlet microtubules surrounding a central tubule) are adjacent to the spindle pole plaques. (B) A network of intermediate filaments underlies the length of the inner membrane complex. (C) Actin is localized between the plasma membrane and inner membrane complex. Greater than 98% of actin is in the monomeric form. Polymerized actin (microfilaments) are only visualized after treatment with the stabilizing drug jasplakinolide. Recent differential extraction data indicates that myosin is associated with the inner membrane complex and actin is associated with the plasma membrane. |
||
Go to:
Main lab page
Research in our laboratory
Publications